My name is Ingrid Chanca and I am a physicist. I am currently pursuing my PhD at the Max Planck Institute for Biogeochemistry, in Jena, Germany in the ATTO project and at the Physics Department of the Universidade Federal Fluminense, in Niterói, Rio de Janeiro, Brazil. For my research, I am particularly interested in radiocarbon, a radioisotope of carbon. Already since the 2nd year of my Bachelor course, I have been working in the Radiocarbon Laboratory of the Universidade Federal Fluminense and I still collaborate with colleagues there through the ATTO project.
Radiocarbon dating
Radiocarbon is produced naturally in the upper atmosphere of Earth when extragalactic particles in the form of cosmic rays collide with the most abundant element of our atmosphere: nitrogen. As a radioactive element, it decays, which means that it emits radiation to become stable. We know that every 5700 years half of the amount of radiocarbon in living tissue will disappear due to this decay. That means, we can measure the concentration of radiocarbon in any sample and estimate how old that sample is or how much time has passed since some event. Radiocarbon works for us like a chronometer. This application is well known for its use as a dating tool. For example, scientists can use it to figure out when the first Homo sapiens on the American continent appeared, I mean…, died.
But radiocarbon is not only useful for events in the past. In my PhD project, I use radiocarbon to get insights into the transit time and age of the carbon in the Amazon rainforest. In order to mitigate global warming, it is important not only to know if the forest can store large amounts of CO2 but also for how long. Therefore, it is essential that we are able to accurately estimate those times.
Measuring radiocarbon from atmospheric CO2
That’s the basic theory. In practice, we have to take some extra steps.
First, we collect samples in the field, for examples soils, plant leaves or even some liters of air. We want to quantify their radiocarbon contents, so we have to make sure that we measure only the carbon we are interested in, not radiocarbon from some dust from the lab, for example.
The idea behind it is this: On the one hand, we measure the radiocarbon signal in the CO2 in the air, which plants capture during photosynthesis. On the other hand, we measure the radiocarbon signal in parts of the plant, and in their respiration. The difference of those signals will help us to estimate the time that passed from when the carbon entered the leaves until it is released through respiration. We also have to convert the sample into graphite. Because we use mass spectrometers coupled to particle accelerators, it is easier for our machines to accelerate carbon in graphite form.
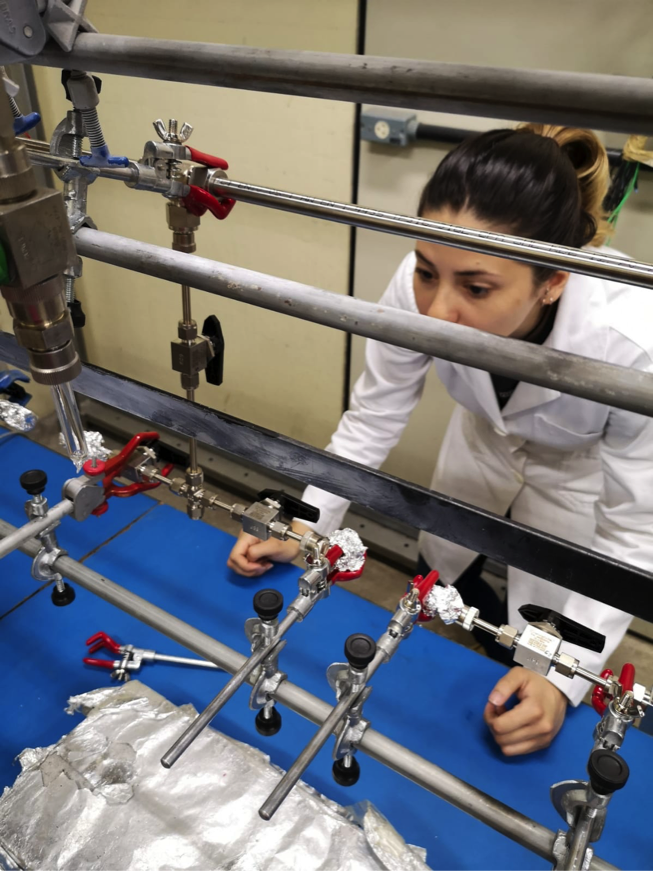
To achieve a sample that is both clean and convertible to graphite, we use a purification system. Especially for air samples from ATTO, I have built the GASPS, a Gas Samples’ Purification System.
A demanding task
Thus, to measure accurately the radiocarbon in the CO2 from the air, we first collect a sample of air in a flask of 3L volume and isolate the CO2 using GASPS. It sounds simple, but remember we don’t want to “contaminate” our samples of air from the Amazon rainforest with air from the laboratory. So, GASPS must be under vacuum, more precisely under 1 mTorr pressure, i.e., a millionth of the standard atmospheric pressure.
Then we want to separate the CO2 from the other gases in the air sample. For this, we can use cryogenic traps. First, we use frozen CO2 (popularly known as dry ice) to freeze the water vapor at -78 °C. The second cryogenic trap is liquid nitrogen (-196 °C), which freezes the CO2, allowing us to pump out the other gases such as nitrogen, oxygen, etc. Then, we transfer the pure CO2 into tubes with graphitization reagents. Finally, the tubes are sealed and can later go to the oven to ignite the reaction that will convert the CO2 to graphite.
Building the Gas Samples’ Purification System GASPS
Building this purification system was a challenge. At first, we had to draw the system to cover all the demands I just described. We want the entire system under vacuum but we will insert a flask full of over-pressurized air. So, we need to control the amount of gas flowing through the system to guarantee the vacuum pump will keep working.
Then we had to obtain all the right pieces. This included stainless steel tubes, valves and connectors for systems under vacuum, pressure sensors, a mass flow controller, glass tubes for the cryogenic traps, and a vacuum pump. Because we built the system from the scratch, this included cutting the tubes by hand. That was a very time demanding, but fun process. After we cut, adjusted, cleaned and baked all the pieces, we could finally put the system up. It ended up being 2 m long and 50 cm high. Once it was all up, we made some final adjustments and checked for leakages.
GASPS is the first purification line completely dedicated to air samples for radiocarbon-accelerator mass spectrometry (14C-AMS) built in our laboratory in Rio. It is going to allow us to extract CO2 from about 6 air samples a day! For such a delicate and hard process, it represents a great achievement for the only radiocarbon facility in South America. Now we can measure lots of samples and hopefully learn a lot more about the transit of carbon in the Amazon rainforest.